What Is The Function Of A Buffer What Is A Buffer Made From . what is buffer in chemistry? buffers made from weak bases and salts of weak bases act similarly. — how a buffer works. — a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Either a weak acid plus a salt derived from that weak acid, or a weak. When you add an acid to a buffer, the conjugate base. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: For example, in a buffer containing nh 3 and nh 4. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. Buffers function through a process of chemical equilibrium. A solution whose ph is not altered to any great extent by the addition of small quantities of.
from classnotes.org.in
— how a buffer works. When you add an acid to a buffer, the conjugate base. For example, in a buffer containing nh 3 and nh 4. A solution whose ph is not altered to any great extent by the addition of small quantities of. buffers made from weak bases and salts of weak bases act similarly. — a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: what is buffer in chemistry? To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. Buffers function through a process of chemical equilibrium.
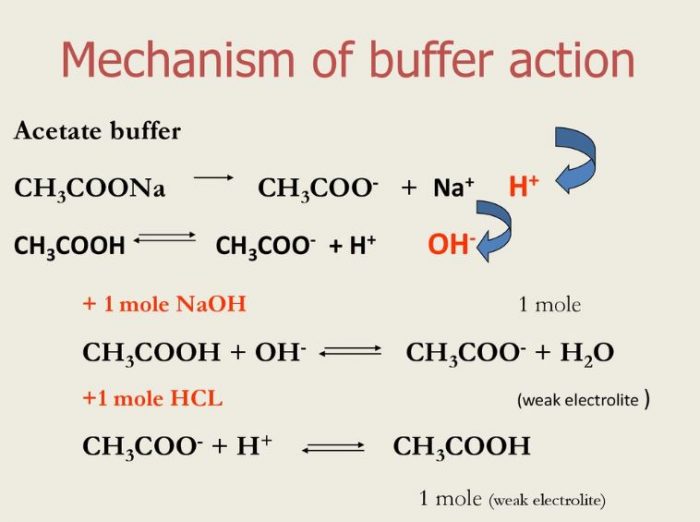
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium
What Is The Function Of A Buffer What Is A Buffer Made From — a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. what is buffer in chemistry? Either a weak acid plus a salt derived from that weak acid, or a weak. A solution whose ph is not altered to any great extent by the addition of small quantities of. — a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. When you add an acid to a buffer, the conjugate base. buffers made from weak bases and salts of weak bases act similarly. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. — how a buffer works. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: For example, in a buffer containing nh 3 and nh 4. Buffers function through a process of chemical equilibrium.
From www.youtube.com
Tristate buffer what is tristate buffer inverting non inverting active low high buffer What Is The Function Of A Buffer What Is A Buffer Made From buffers resist dramatic changes in ph by being composed of certain pairs of solutes: buffers made from weak bases and salts of weak bases act similarly. Either a weak acid plus a salt derived from that weak acid, or a weak. A solution whose ph is not altered to any great extent by the addition of small quantities. What Is The Function Of A Buffer What Is A Buffer Made From.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation What Is The Function Of A Buffer What Is A Buffer Made From Buffers function through a process of chemical equilibrium. buffers made from weak bases and salts of weak bases act similarly. Either a weak acid plus a salt derived from that weak acid, or a weak. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. buffers resist dramatic changes. What Is The Function Of A Buffer What Is A Buffer Made From.
From www.osmosis.org
Making buffer solutions Osmosis What Is The Function Of A Buffer What Is A Buffer Made From Either a weak acid plus a salt derived from that weak acid, or a weak. A solution whose ph is not altered to any great extent by the addition of small quantities of. Buffers function through a process of chemical equilibrium. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: To illustrate the function. What Is The Function Of A Buffer What Is A Buffer Made From.
From study.com
Buffer System in Chemistry Definition & Overview Video & Lesson Transcript What Is The Function Of A Buffer What Is A Buffer Made From — how a buffer works. For example, in a buffer containing nh 3 and nh 4. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. A solution whose ph is not altered to any great extent by the addition of small quantities of. buffers resist dramatic changes in. What Is The Function Of A Buffer What Is A Buffer Made From.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Is The Function Of A Buffer What Is A Buffer Made From For example, in a buffer containing nh 3 and nh 4. — a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. what is buffer in chemistry? Either a weak acid plus a salt derived from that weak acid, or a weak. To illustrate the function of a buffer. What Is The Function Of A Buffer What Is A Buffer Made From.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Is The Function Of A Buffer What Is A Buffer Made From buffers made from weak bases and salts of weak bases act similarly. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. — how a buffer works. A solution whose ph is not altered to any great extent by the addition of small quantities of. Buffers function through a. What Is The Function Of A Buffer What Is A Buffer Made From.
From www.youtube.com
Buffer action in the blood YouTube What Is The Function Of A Buffer What Is A Buffer Made From — how a buffer works. Either a weak acid plus a salt derived from that weak acid, or a weak. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: what is buffer in chemistry? When you add an acid to a buffer, the conjugate base. — a buffer is a solution. What Is The Function Of A Buffer What Is A Buffer Made From.
From dxopbwbtw.blob.core.windows.net
How Do Buffers Work In Blood at Carter Nolan blog What Is The Function Of A Buffer What Is A Buffer Made From — how a buffer works. A solution whose ph is not altered to any great extent by the addition of small quantities of. what is buffer in chemistry? Buffers function through a process of chemical equilibrium. When you add an acid to a buffer, the conjugate base. For example, in a buffer containing nh 3 and nh 4.. What Is The Function Of A Buffer What Is A Buffer Made From.
From www.youtube.com
Buffer Solutions YouTube What Is The Function Of A Buffer What Is A Buffer Made From what is buffer in chemistry? buffers made from weak bases and salts of weak bases act similarly. When you add an acid to a buffer, the conjugate base. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts. What Is The Function Of A Buffer What Is A Buffer Made From.
From dxonofogy.blob.core.windows.net
Buffer Solutions Laboratory at Danny Wetzler blog What Is The Function Of A Buffer What Is A Buffer Made From When you add an acid to a buffer, the conjugate base. Buffers function through a process of chemical equilibrium. what is buffer in chemistry? buffers made from weak bases and salts of weak bases act similarly. Either a weak acid plus a salt derived from that weak acid, or a weak. buffers resist dramatic changes in ph. What Is The Function Of A Buffer What Is A Buffer Made From.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 What Is The Function Of A Buffer What Is A Buffer Made From what is buffer in chemistry? For example, in a buffer containing nh 3 and nh 4. — a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. — how a buffer works. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of. What Is The Function Of A Buffer What Is A Buffer Made From.
From www.youtube.com
What is Buffer ? Why Buffer and TriState Buffers are used in Digital Circuits ? YouTube What Is The Function Of A Buffer What Is A Buffer Made From — how a buffer works. Either a weak acid plus a salt derived from that weak acid, or a weak. Buffers function through a process of chemical equilibrium. When you add an acid to a buffer, the conjugate base. — a buffer is a solution that can resist ph change upon the addition of an acidic or basic. What Is The Function Of A Buffer What Is A Buffer Made From.
From saylordotorg.github.io
Buffers What Is The Function Of A Buffer What Is A Buffer Made From Either a weak acid plus a salt derived from that weak acid, or a weak. — how a buffer works. buffers made from weak bases and salts of weak bases act similarly. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. buffers resist dramatic changes in ph. What Is The Function Of A Buffer What Is A Buffer Made From.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its action. YouTube What Is The Function Of A Buffer What Is A Buffer Made From buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A solution whose ph is not altered to any great extent by the addition of small quantities of. what is buffer in chemistry? Either a weak acid plus a salt derived from that weak acid, or a weak. To illustrate the function of a. What Is The Function Of A Buffer What Is A Buffer Made From.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Is The Function Of A Buffer What Is A Buffer Made From Buffers function through a process of chemical equilibrium. — a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffers made from weak bases and salts of weak bases act similarly. When you add an acid to a buffer, the conjugate base. — how a buffer works. Either. What Is The Function Of A Buffer What Is A Buffer Made From.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is The Function Of A Buffer What Is A Buffer Made From A solution whose ph is not altered to any great extent by the addition of small quantities of. buffers made from weak bases and salts of weak bases act similarly. When you add an acid to a buffer, the conjugate base. For example, in a buffer containing nh 3 and nh 4. — how a buffer works. . What Is The Function Of A Buffer What Is A Buffer Made From.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses What Is The Function Of A Buffer What Is A Buffer Made From A solution whose ph is not altered to any great extent by the addition of small quantities of. When you add an acid to a buffer, the conjugate base. buffers made from weak bases and salts of weak bases act similarly. — how a buffer works. Either a weak acid plus a salt derived from that weak acid,. What Is The Function Of A Buffer What Is A Buffer Made From.
From dxobwrvez.blob.core.windows.net
Common Buffers In Biochemistry at Mario Ross blog What Is The Function Of A Buffer What Is A Buffer Made From — a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. what is buffer in chemistry? buffers resist dramatic changes in ph by being composed of certain pairs of solutes: For example, in a buffer containing nh 3 and nh 4. To illustrate the function of a buffer. What Is The Function Of A Buffer What Is A Buffer Made From.